Immunomechanics & Mechano-immunology
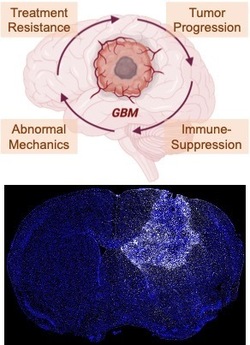
New fields of research at the intersection of tissue mechanics and immunology have emerged in recent years that are analogous to biomechanics and mechanobiology. Immunomechanics research is designed to determine and perturb the mechanical forces and resulting deformations that immune cells experience in physiological or pathophysiological settings, as well as the mechanical forces that immune cells themselves can exert. Mechano-immunology examines how tissue mechanics affect immune cell phenotype and function. Because mechanopathologies are a hallmark of solid tumors, we study the impact of mechanical properties and forces on pro-tumor and anti-tumor immune responses within and surrounding the tumor microenvironment. We utilize multi-scale models and tools to either measure or apply mechanical forces from the single-cell level up to tissue length scales in cell cultures, organotypic 3-D tissues, and mouse models of cancer. By revealing and targeting these mechanical- and immune-related vulnerabilities – e.g., with FDA-approved agents for rapid translational potential – we can re-engineer the tumor microenvironment to sensitize it to anti-cancer treatment such as immunotherapy.
Publications:
Burchett A.A., Siri S., Li J., Lu X., and Datta M.# “Novel 3-D macrophage spheroid model reveals reciprocal regulation of immunomechanical stress and mechano-immunological response," submitted. Preprint available on bioRxiv. (# = corresponding author.)
Datta M.*,#, Via L.E.*, Dartois V.*, Weiner D.M., Zimmerman M., Kaya F., Walker A.M., Fleegle J.D., Raplee I.D., McNinch C., Zarodniuk M., Kamoun W.S., Yue C., Kumar A.S., Subudhi S., Xu L., Barry C.E., and Jain R.K.#“Normalizing granuloma vasculature and matrix improves drug delivery and reduces bacterial burden in tuberculosis-infected rabbits,” Proceedings of the National Academy of Sciences, E-pub ahead of print: https://www.pnas.org/doi/abs/10.1073/pnas.2321336121. (* = equal contributions; # = corresponding authors.)Featured in Notre Dame News, EurekAlert!, Medical Xpress, Science Daily, and Genetic Engineering & Biotechnology News.
Shanmugarajan K., Lee S., Subudhi S., Kumar A.S., Amoozgar Z., Posada J., Lindeman N., Lei P., Duquette M., Roberge S., Huang P., Andersson P., Datta M., Munn L.L., Fukumura D., and Jain R.K. “Wnt inhibition alleviates resistance to immune checkpoint blockade therapy in glioblastoma,” submitted. Preprint available on Research Square.
Chen J., Amoozgar Z., Liu X., Aoki S., Liu Z., Shin S., Matsui A., Pu Z., Lei P., Zhu L., Ruan Z., Shi L., Staiculescu D., Inoue K., Munn L.L., Fukumura D., Huang P. Bardeesy N., Ho W.J., Jain R.K., and Duda D.G. (2024) “Reprogramming intrahepatic cholangiocarcinoma immune microenvironment by chemotherapy and CTLA-4 blockade enhances anti-PD1 therapy,” Cancer Immunology Research, E-pub ahead of print: https://doi.org/10.1158/2326-6066.CIR-23-0486.
Iorgulescu J.B., Ruthen N.*, Ahn R.*, Panagioti E.*, Gokhale P.*, Neagu M., Speranza M.C., Eschle B.K., Soroko K.M., Piranlioglu R., Datta M., Krishnan S., Yates K.B., Baker G., Jain R.K., Suvà M.L., Neuberg D., White F.M., Chiocca E.A., Freeman G.J., Sharpe A.H., Wu C.J., and Reardon D.A. (2023) “Antigen presentation deficiency, mesenchymal differentiation, and resistance to immunotherapy in the murine syngeneic CT2A tumor model,” Frontiers in Immunology, E-pub ahead of print: https://doi:10.3389/fimmu.2023.1297932.
Zarodniuk M., Steele A., Lu X., Li J., and Datta M.# “CNS tumor stroma transcriptomics identify perivascular fibroblasts as predictors of immunotherapy resistance in glioblastoma patients,” npj Genomic Medicine, E-pub ahead of print: https://www.nature.com/articles/s41525-023-00381-w. (# = corresponding author.)
Holen L.*, Onwudiwe K.*, Najera J., Zarodniuk M., Obayemi J.D., Soboyejo W.O., and Datta M.# (2023) “Shear assay protocol for the determination of single-cell material properties,” Journal of Visualized Experiments, E-pub ahead of print: https://www.jove.com/t/65333/shear-assay-protocol-for-determination-single-cell-material. (* = equal contributions; # = corresponding author.)
Zhang S.*, Regan K.*, Najera J.*, Chu V., Grinstaff M.W., Datta M.#, and Nia H.T.# “The peritumor microenvironment: physics and immunity,” Trends in Cancer, E-pub ahead of print: https://authors.elsevier.com/a/1h1a98Z12yTuSQ. (* = equal contributions)
Dong X.*, Ren J.*, Amoozgar Z., Lee S., Datta M., Roberge S., Duquette M., Fukumura D., and Jain R.K. (2023) “Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice,” Journal for ImmunoTherapy of Cancer, 11: e005583, E-pub ahead of print: https://jitc.bmj.com/content/11/3/e005583.
Datta M.#, Chatterjee S., Perez E.M., Gritsch S., Roberge S., Duquette M., Chen I.X., Naxerova K., Kumar A.S., Ghosh M., Emblem K.E., Ng M.R., Ho. W.W., Kumar P., Krishnan S., Dong X., Speranza M.C., Neagu M.R., Reardon D.A., Sharpe A.H., Freeman G.J., Suva M.L.#, Xu L.#, Jain R.K.# (2023) “Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma,” E-pub ahead of print: https://www.pnas.org/eprint/MP69MI2GBHTY9VJKDSBQ/full.
Onwudiwe K.*, Najera J.*, Siri S., and Datta M.# (2022) "Do tumor mechanical stresses promote cancer immune escape?" Cells, 11: 3840. (* = equal contributions.)
Datta M. (2022) “Studying the tumor microenvironment under pressure,” Immuno-Oncology Insights, 3: 339-344.
Ho W.W., Gomes-Santos I.L., Talele N.P., Aoki S., Datta M., Kawagushi K., Ren J., Liu H. Chen I.X., Nojiri T., Chatterjee S., Zhao Y., Millar D.G., Clark J.W., Cobbold M., Pittet M.J., Fukumura D., and Jain R.K. (2021) “Orthotopic liver metastatic mouse models of mismatch repair-proficient colorectal cancer predict clinical inefficacy of immune checkpoint blockade,” Proceedings of the National Academy of Sciences, E-pub ahead of print: https://www.pnas.org/content/118/45/e2105323118.
Wu L., Vasilijic S., Sun Y., Chen J., Landegger L.D., Zhang Y., Zhou W., Ren J., Yin Z., Ho W.W., Zhang N., Gao X., Datta M., Early S., Brown A., Sagers J.E., Muzikansky A., Zhang L., Stemmer- Rachamimov A., Plotkin S.R., Jain R.K., Stankovic K.M., and Xu L. (2021) “Losartan prevents tumor-induced hearing loss and augments radiation efficacy by normalizing the tumor microenvironment in NF2 schwannoma models,” Science Translational Medicine, E-pub ahead of print: https://stm.sciencemag.org/content/13/602/eabd4816.abstract. Featured in EurekAlert, Scienmag, Medical Xpress, GEN, and Hearing Health & Technology Matters.
Nia H.T.*, Datta M.*, Seano G.*, Ho W.W., Roberge S., Huang P., Munn L.L. and Jain R.K. (2020) “In vivo compression and imaging in mouse brain to measure the effects of solid stress,” Nature Protocols, 15: 2021-2340. (* = equal contributions.) Featured in Nature Community.
Flores-Toro J.A., Luo D., Gopinath A., Sarkisian M., Campbell J.J., Charo I.F., Singh R., Schall T.J., Datta M., Jain R.K., Mitchell D.A., and Harrison J.K. (2019) “CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas,” Proceedings of the National Academy of Sciences, 117: 1129-1138. Featured in Medical Xpress and EurekAlert.
Shigeta K*., Datta M.*, Hato T.*, Kitahara S.*, Chen I.X., Mamessier E. Matsui A., Ramijiawan R.R., Aoki S., Ochiai H., Bardeesy N., Huang P., Cobbold M., Zhu A.X., Jain R.K., and Duda D.G. (2019) “Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances anti-tumor immune responses in HCC,” Hepatology, 7: 1247-1261. (* = equal contributions.)
Zhao Y. Cao J., Melamed A., Worley M., Gockley A., Jones D., Nia H.T., Zhang Y., Stylianopoulos T., Kumar A.S., Mpekris F., Datta M., Sun Y., Wu L., Gao X., Yeku O., del Carmen M., Spriggs D.R., Jain R.K., and Xu L. (2019) “Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma,” Proceedings of the National Academy of Science, 116: 2210-2219. Featured in New England Journal of Medicine Journal Watch, Medical News Bulletin, Ovarian Cancer News Today, and Medical Xpress.
Nia H.T.*, Datta M.*, Seano G., Munn L.L., and Jain R.K. (2018) “Quantifying solid stress and elastic energy from excised or in situ tumors,” Nature Protocols 13: 1091-1105. (* = equal contributions.)
Peterson T.E.*, Kirkpatrick N.D.*, Huang Y.*, Farrar C., Marijt K.A., Kloepper J., Datta M., Amoozgar Z., Seano G., Jung K., Kamoun W.S., Vardam T., Snuderl M., Goveia J., Chatterjee S., Batista A., Muzikansky A., Leow C.C., Xu L., Batchelor T.T., Duda D.G., Fukumura D., and Jain R.K. (2016) “Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages,” Proceedings of the National Academy of Sciences 113: 4470-4475. (* = equal contributions.) Featured in Science Daily.
Kloepper J.*, Riedemann L.*, Amoozgar Z.*, Seano G., Susek K.H., Yu V., Dalvie N., Amelung R.L., Datta M., Song J.W., Askoxylakis V., Taylor J.W., Lu-Emerson C., Batista A., Kirkpatrick N.D., Snuderl M., Muzikansky A., Stubenrauch K.G., Wakimoto H., Xu L., Munn L.L., Duda D.G., Fukumura D., Batchelor T.T., and Jain R.K. (2016) “Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival,” Proceedings of the National Academy of Sciences 113: 4476-4481. (* = equal contributions.) Featured in Science Daily.